The Therapeutic Applications of
Adenosine Receptor Types 2A and 2B
Abstract
Adenosine is mostly produced by catabolism of adenosine triphosphate (ATP). Adenosine has many different physiological functions. In lecture we learned that it is an inhibitory neurotransmitter and caffeine works by blocking its effects. Adenosine receptors, its agonists and antagonists have plenty more effects, many of them could be manipulated for therapeutic effects. There are four types of adenosine receptors: A1, A2A, A2B, and A3. In this paper, I will focus on the A2A and A2B receptors, (collectively called A2 adenosine receptors) the effects of its agonists and antagonists, and their potential medicinal use.
A2 adenosine receptors are present in both the central nervous system (CNS) and the peripheral nervous system (PNS). In the central nervous system, A2A adenosine receptors have neuroprotective properties. Its activation prevents or reduces Alzheimerís disease, Schizophrenia, and cocaine addiction. Blocking A2A adenosine receptors in the CNS protects against Dyskinesia, Parkinsonís disease, and Huntingtonís disease. In the peripheral nervous system, activation of A2A adenosine receptors reduces inflammation and ischemic injury, advances retinal vascular development, and relaxes vessels. A2B adenosine receptors work with A2A receptors in advancing retinal blood vessel growth and relaxing the vessels. Activation of A2B receptors are also associated with asthma, implying that antagonists could be used to treat asthma.
Activation of A2 Receptors in the CNS
A2A receptors are located in the neocortex, olfactory bulb, striatumpallidal GABAergic neurons, hippocampus, thalamus, and nucleus tractus solitarius. (Ribeiro et al. 2003)
 Fig. 1. Distribution of high affinity adenosine receptors (A1, A2A and human A3) in the main regions of the central nervous system where adenosine has been proposed to interfere with brain dysfunctions and disease. High levels of expression are indicated by bigger alphabets. (Taken from Ribeiro et al. 2003)
Fig. 1. Distribution of high affinity adenosine receptors (A1, A2A and human A3) in the main regions of the central nervous system where adenosine has been proposed to interfere with brain dysfunctions and disease. High levels of expression are indicated by bigger alphabets. (Taken from Ribeiro et al. 2003)
First letís look at the effects of activating A2A adenosine receptors in the CNS. The activation of A2A receptors facilitate acetylcholine release which is useful in treating Alzheimerís disease (Sebastiao & Ribeiro 1996). Alzheimerís disease is partly due to the reduced level of the neurotransmitter acetylcholine, which is essential for memory and learning. By increasing the amount of acetylcholine in the synapse, Alzheimerís disease can be alleviated.
A2A adenosine receptorsí antagonisticly interact with dopamine receptor type 2 (D2) in the striopallidal GABAergic neurons (Akhondzadeh et al. 2000). Haloperidol is used to treat schizophrenia. Dipyridamole, an adenosine uptake inhibitor, which increases extracellular adenosine concentration, when used with haloperidol was significantly more effective as an anti-psychotic treatment than the use of haloperidol alone. Therefore, activation of A2A receptors by adenosine can be used to treat schizophrenia as well.
Activation of A2A adenosine receptors also reduces cocaine self-administration in rats (Knapp et al. 2001). Treatment of CGS 21680, an A2A adenosine receptor selective agonist was given to rats that had free access to intravenous cocaine at 0.6 mg/kg/infusion and resulted in decreased cocaine use per session in rats. The use of adenosine also reduces morphine withdrawal (Capasso & Loizzo 2001).
A2B adenosine receptor interacts with netrin-1, a protein that regulate axon growth (Corset et al 2000). Netrin-1 binds to A2B receptor and activates it, which then leads to cyclic AMP production. Thus, A2B adenosine receptors could affect neuronal assembly.
Antagonists of A2 Receptors in the CNS
Parkinsonís disease is characterized with impaired ability to initiate movements. It can be treated with L-DOPA. But L-DOPA can induce dyskinesia, an impaired ability to control movements, resulting in tremor and spasmodic motions. Treatment with KW-6002, an A2A adenosine receptor antagonist, produced much relief of motor disability without spasmodic motions (Lundblad et al. 2003). Although treatment of KW-6002 and L-DOPA together had better improvement in Parkinsonís disease, it also induced dykinesia to the same degree as treatment of L-DOPA alone. Experiment was conducted in rats with 6-hydroydopamine lesions. Concluded was that treatment of A2A adenosine receptor antagonist alone for Parkinsonís disease can alleviate motor disability without inducing dyskinesia. The mechanism of how this works could be contributed to the monoamine oxidase B (MAO B) inhibition by A2A antagonists (Petzer et al. 2003). MAO B degrades dopamine, a lack of which is the cause of Parkinsonís disease. By inhibiting MAO B, A2A adenosine receptor antagonists increases levels of dopamine and thus lessen Parkinsonís disease. This study examined (E)-8-styrylxanthinyl derived A2A adenosine receptor antagonists. All showed antiparkinsonian activities. MAO B also catalyze the conversion of neurotoxin 1-methyl-4-phenul-1,2,3,6-tetrahydropyridine (MPTP) into MPP+, a mitochondrial toxin and a dopaminergic neuron toxin. MPP+ is taken up by dopamine transporters and kills cells by interfering with oxidative-phosphrylation (Presti lecture 2003). By inhibiting MAO B, A2A adenosine receptor antagonists acts as a neuroprotective agent.
The A2A adenosine receptor antagonist SCH 58261 protects against Huntingtonís disease (Popoli et al. 2002). Rats were given SCH 58261 intraperitoneally at either 0.01 mg/kg or 1 mg/kg twenty minutes before quinolinic acid bilaterally injected into striatum, which was used to induce excitotoxicity. Those rats treated with SCH 58261 at low doses were protected from motor activity and striatal gliosis. More experiments showed that the effect mainly came from inhibiting quinolinic acid from causing glutamate outflow, thus inhibiting its excitotoxic effects presynaptically. Since Huntingtonís disease is caused by degeneration of the cells in striatum, if A2A adenosine receptor antagonists can protect against that, then it could be a treatment for Huntingtonís disease.
A2A and A2B Receptors in the PNS
Treating autoimmune diseases requires the inhibition of inflammation. A2A adenosine receptor is a sensor of tissue damage caused by too much inflammation (Sitkovsky 2003). Tissue damaged this way and hypoxia leads to adenosine buildup, which in turn leads to activation of A2A adenosine receptors and elevation of cyclic AMP. Agonists of A2A adenosine receptors are immunosuppressive while antagonists are inflammation-enhancing. If we can figure out a way to selective enhance inflammation, we can use it to improve treatments of immunotherapies of tumors and to develop vaccines. Thus the agonists of A2A adenosine receptors can be used to inhibit inflammation to treat autoimmune diseases and sepsis while antagonists can be used for selectively enhanced inflammation and targeted tissue damage.
Ischemic injury is caused by not having enough oxygen. This could happen especially after surgery. For example, renal ischemic reperfusion injury has high death rates during the postoperative period. Adenosine, as an A2A adenosine receptor agonist works in many organ systems to prevent ischemic injury, such as the heart, liver, brain, and kidney (Lee & Emala 2002). In this particular study, A2A adenosine receptor agonists minimized harsh ATP depletion injury in cultured human proximal tubule (HK-2) cells. The selective A2A adenosine receptor agonist used was 1nM Ė 10 žM of 4-([N-ethyl-5í-carbamoyadenos-2-yl]-aminoethyl)-phenylpropionic acid (CGS-21680) for 30 minutes.
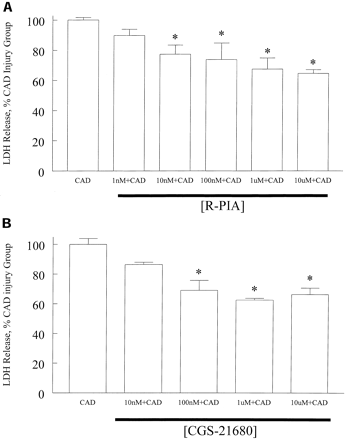 Figure 2. The A1 adenosine receptor agonist (PIA; panel A; n = 6) and the A2a adenosine receptor agonist (CGS; panel B; n = 6) attenuate severe ATP depletion-mediated HK-2 cell injury (CAD; n = 6) in dose-dependent manners. Cell injuries were quantified by measuring LDH released into the culture media from cells treated with 1 nM to 10 ĶM PIA or 0.01 to 10 ĶM CGS or vehicle for 30 min before the addition of 10 ĶM antimycin A, 10 mM 2-deoxyglucose, 2 ĶM and calcium ionophore (CAD, n = 6) for 2 h. * P < 0.05 versus severe ATP depletion injury group (CAD). Error bars represent 1 SEM. (Taken from Lee & Emala 2002)
Figure 2. The A1 adenosine receptor agonist (PIA; panel A; n = 6) and the A2a adenosine receptor agonist (CGS; panel B; n = 6) attenuate severe ATP depletion-mediated HK-2 cell injury (CAD; n = 6) in dose-dependent manners. Cell injuries were quantified by measuring LDH released into the culture media from cells treated with 1 nM to 10 ĶM PIA or 0.01 to 10 ĶM CGS or vehicle for 30 min before the addition of 10 ĶM antimycin A, 10 mM 2-deoxyglucose, 2 ĶM and calcium ionophore (CAD, n = 6) for 2 h. * P < 0.05 versus severe ATP depletion injury group (CAD). Error bars represent 1 SEM. (Taken from Lee & Emala 2002)
We can see from fig. 2B that when CGS-21680 is given before ATP depletion by antimycin A, deoxyglucose and calcium ionophore, protect against ischemic injury.
Adenosine is also abundant in the inner retina during vascular development in dogs (Lutty & McLeod 2003). A2A adenosine receptors exists on blood vessel endothelial cells and their precursors, thus adenosine must be crucial for vascular development in the retina. This study used oxygen-induced retinopathy (OIR) as a model for human retinopathy of prematurity (ROP). Vascular development was stopped by exposing retina to high oxygen, which resulted in a quick adenosine decrease. When returned to room air, a much elevated A2A adenosine receptor expression was found in the dog, resulting in a vasoproliferative stage which can lead to astrogliosis and hold back anterior vascular development. Since adenosine and its corresponding A2A receptor are important in retinal vasculature development, one of the A2 adenosine recetors could be used therapeutically to inhibit neovascularization in OIR.
This was confirmed by a study done in mouse (Mino et al. 2001). Adenosine is the eyeís tool of compensation when there is not enough oxygen or nutrients. By blocking it receptor, in this case, A2B adenosine receptor, we can inhibited OIR induced neovascularization and thus could treat eye diseases that involve excessive growth of retinal tissues.
Activation of A2A adenosine receptor by adenosine in endothelial cells of the porcine carotid artery stimulates the release of nitric oxide which causes vasodilation. Activation of A2A and A2B adenosine increases cyclic AMP accumulation, result in vasorelaxation.
A2B adenosine receptors exist in the human lung mast cells (Feoktistov & Biaggioni 1996). Inhalation of adenosine causes bronchoconstriction in asthmatics. Enprofylline, a xanthine used to treat asthma, was found to block the A2B adenosine receptor with a Ki of 7 žM. This finding sustains the idea that activation of A2B receptors lead to asthma, thus its selective antagonists should have therapeutic value in treating asthma.
Conclusion
In conclusion, adenosine receptors have many functions and effects in both the central and the peripheral nervous system. A2 receptors in general have neuroprotective effects as observed in prevention of Alzheimers and dyskinesia. There are not as much information on A2B adenosine receptors as there are A2A receptors but one highly selective antagonist for A2B have been identified and that is enprofylline which treats asthma. More scientific research still needs to be done about the adenosine receptors, especially the less well known ones. They will probably give us answers to help us improve our treatment for many diseases.
 Fig. 1. Distribution of high affinity adenosine receptors (A1, A2A and human A3) in the main regions of the central nervous system where adenosine has been proposed to interfere with brain dysfunctions and disease. High levels of expression are indicated by bigger alphabets. (Taken from Ribeiro et al. 2003)
Fig. 1. Distribution of high affinity adenosine receptors (A1, A2A and human A3) in the main regions of the central nervous system where adenosine has been proposed to interfere with brain dysfunctions and disease. High levels of expression are indicated by bigger alphabets. (Taken from Ribeiro et al. 2003)
 Figure 2. The A1 adenosine receptor agonist (PIA; panel A; n = 6) and the A2a adenosine receptor agonist (CGS; panel B; n = 6) attenuate severe ATP depletion-mediated HK-2 cell injury (CAD; n = 6) in dose-dependent manners. Cell injuries were quantified by measuring LDH released into the culture media from cells treated with 1 nM to 10 ĶM PIA or 0.01 to 10 ĶM CGS or vehicle for 30 min before the addition of 10 ĶM antimycin A, 10 mM 2-deoxyglucose, 2 ĶM and calcium ionophore (CAD, n = 6) for 2 h. * P < 0.05 versus severe ATP depletion injury group (CAD). Error bars represent 1 SEM. (Taken from Lee & Emala 2002)
Figure 2. The A1 adenosine receptor agonist (PIA; panel A; n = 6) and the A2a adenosine receptor agonist (CGS; panel B; n = 6) attenuate severe ATP depletion-mediated HK-2 cell injury (CAD; n = 6) in dose-dependent manners. Cell injuries were quantified by measuring LDH released into the culture media from cells treated with 1 nM to 10 ĶM PIA or 0.01 to 10 ĶM CGS or vehicle for 30 min before the addition of 10 ĶM antimycin A, 10 mM 2-deoxyglucose, 2 ĶM and calcium ionophore (CAD, n = 6) for 2 h. * P < 0.05 versus severe ATP depletion injury group (CAD). Error bars represent 1 SEM. (Taken from Lee & Emala 2002)